Presenting significant potential for advancements in precision medicine
Heralding a new era in personalized healthcare this innovative model holds promise for personalized medical interventions by enabling the generation of neurovascular structures tailored to individual patients’ needs.
A groundbreaking prototype has been developed to generate neurovascular tissues or organoids from a patient’s own blood, offering promising prospects for precision medicine. This innovative model enables the creation of masses of neurovascular tissues, termed neurovascular organoids or embryoids (NVOEs), which can aid in the study of impaired brain function and development. By analyzing neuroimaging scans alongside alterations in blood supply, researchers can delve into the complexities of brain conditions. By sidestepping traditional approaches reliant on external genetic manipulation or morphogenic supplementation, this prototype represents a paradigm shift in tissue engineering and regenerative medicine.
The realm of neural organoids is rapidly evolving, presenting opportunities for enhanced comprehension of brain functions, disease modeling, drug discovery, and transplantation alternatives. Traditional neuronal organoids often lack vascularization and rely on genetic manipulation, posing limitations. In response, a novel approach involving the co-culturing of blood vessel organoids with cerebral organoids has been proposed but remains labor-intensive and costly.
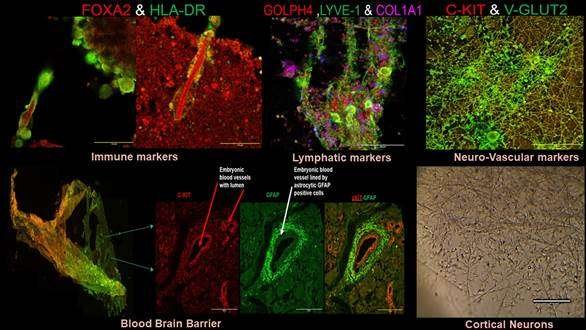
Addressing this challenge, researchers at the Post Graduate Institute of Medical Education & Research (PGIMER) in Chandigarh have devised a prototype for the creation and characterization of self-organizing NVOEs solely from autologous blood, eliminating the need for genetic modification or morphogen supplementation. This approach yields functional vascularized embryoids without guided patterning, offering a cost-efficient solution requiring only autologous plasma and blood cells, without the need for specialized media or growth factors.
Validation of the functional vasculature within neurovascular embryoids was confirmed through the detection of hemoglobin and deoxyhemoglobin signals using the Blood-Oxygen-Level-Dependent (BOLD) signal concept. The implications of this advancement are vast, spanning from studying neurological disease pathways to preclinical neuroimaging and autologous immunotherapies for tumors and autoimmune diseases.
The researchers, supported by funding from the Anusandhan National Research Foundation (ANRF), are in the process of patenting this innovation through the Punjab State Council for Science and Technology. They are applying these models to investigate the genetic underpinnings of neurosensory hearing loss and auditory comprehension challenges in children, aiming to enhance outcomes for cochlear implant recipients by exploring altered central auditory activity pathways.
This prototype holds promise for developing patient-specific models for congenital neurological conditions, including autism, ADHD, auditory neuropathy spectrum disorder (ANSD), Alzheimer’s, and Parkinson’s disease. It also opens avenues for deciphering genetics, testing drugs, and identifying biomarkers for early neurological diseases, offering a transformative approach in the landscape of precision medicine.
At its core, the significance of this advancement extends far beyond its technical intricacies. By enabling the production of neurovascular tissues tailored to individual patients, this prototype holds immense promise for revolutionizing the landscape of medical treatment. No longer constrained by the limitations of standardized approaches, clinicians and researchers alike can now envision a future where therapies are precisely calibrated to meet the unique needs of each patient.
At its core, the significance of this advancement extends far beyond its technical intricacies. By enabling the production of neurovascular tissues tailored to individual patients, this prototype holds immense promise for revolutionizing the landscape of medical treatment. No longer constrained by the limitations of standardized approaches, clinicians and researchers alike can now envision a future where therapies are precisely calibrated to meet the unique needs of each patient. This not only makes the technology more accessible but also facilitates its widespread adoption across diverse healthcare settings.
In conclusion, the development of this prototype represents a watershed moment in the field of medical research. By harnessing the body’s own resources to generate neurovascular tissues tailored to individual patients, it offers a glimpse into a future where healthcare is truly personalized, precise, and patient-centric.
(Story & Pic. Courtesy: PIB)